TOBRA CEF
CEFTAZIDIME AND TOBRAMYCIN FOR INJECTION
For IV use
Tobracef (Ceftazidime and Tobramycin) for injection is combination of Ceftazidime Pentahydrate and Tobramycin Sulphate available as sterile dry powder for reconstitution before use. Ceftazidime and Tobramycin for injection is supplied for IV administration in strength equivalent to 0.560g, 1.12g and 2.18g along with new solvent for injection (SFI) as diluent . The combination dosage form supplied as sterile powder for injection. Composition:
| PRODUCT STRENGTH | EACH VIAL CONTAINS | QUANTITY |
| 560mg | Ceftazidime Pentahydrate USP eqv. to Ceftazidime Tobramycin Sulphate USP eqv. to Tobramycin | 500 mg 60 mg |
| 1.12 g | Ceftazidime Pentahydrate USP eqv. to Ceftazidime Tobramycin Sulphate USP eqv. to Tobramycin | 1000 mg 120 mg |
CLINICAL PARTICULARS Tobracef is indicated for the treatment of patients with infections caused by susceptible strains of the designated organisms in the following disease: -
(1) Lower respiratory tract infections caused by P.aeruginosa, Klebsiella spp, Enterobacter spp, Serratia spp, E.coli, and S.aureus (penicillinase and non-penicillinase producing strains)
(2) Serious central-nervous-system infections (meningitis) caused by susceptible organisms
(3) Intra-abdominal infections, including peritonitis, caused by E. coli, Klebsiella spp, and Enterobacter spp
(4) Skin, bone, and skin structure infections caused by P.aeruginosa, Proteus spp, E. coli, Klebsiella spp, Enterobacter spp, and S.aureus
(5) Complicated and recurrent urinary tract infections caused by P.aeruginosa, Proteus spp (indole-positive and indole-negative),E. coli, Klebsiella spp, Enterobacter spp, Serratia spp, S.aureus, Providencia spp, and Citrobacter spp
(6) Bacterial Septicemia in the pediatric patient and adult caused by. P. aeruginosa, E. coli, and Klebsiella spp
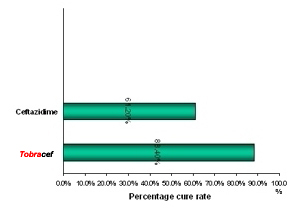
(7) Gynecological Infections Clinical Data Results of clinical Trials in Patients have shown better treatment or cure rate in patients receiving Tobracef (with new solvent) in comparison to Ceftazidime alone (Fig 1).
Fig. 1 Results of clinical trials of Cetazidime-Tobramycin with NEW solvent in comparison with ceftazidime alone.
DRUG INTERACTIONS
Nephrotoxicity has been reported following concomitant administration of cephalosporins with aminoglycoside antibiotics or potent diuretics such as frusemide. Renal function should be carefully monitored, especially if higher dosages of the aminoglycosides are to be administered or if therapy is prolonged, because of the potential nephrotoxicity and ototoxicity of aminoglycosidic antibiotics. Nephrotoxicity and ototoxicity were not noted when Ceftazidime was given alone in clinical trials. Serum and urine specimens for examination should be collected during therapy Serum calcium, magnesium, and sodium should be monitored.
WARNINGS
FLUIDS, IV ANTIHISTAMINES, CORTICOSTEROIDS, PRESSOR AMINES, AND AIRWAY MANAGEMENT, BEFORE THERAPY WITH Tobracef (CEFTAZIDIME AND TOBRAMYCIN) IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEFTAZIDIME AND TOBRAMYCIN, CEPHALOSPORINS, PENICILLINS, OR OTHER DRUGS. IF THIS PRODUCT IS TO BE GIVEN TO PENICILLIN-SENSITIVE PATIENTS, CAUTION SHOULD BE EXERCISED BECAUSE CROSS-HYPERSENSITIVITY AMONG BETA-LACTAM ANTIBIOTICS HAS BEEN CLEARLY DOCUMENTED AND MAY OCCUR IN UP TO 10% OF PATIENTS WITH A HISTORY OF PENICILLIN ALLERGY. IF AN ALLERGIC REACTION TO CEFTAZIDIME-TOBRAMYCIN COMBINATION OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE TREATMENT WITH EPINEPHRINE AND OTHER EMERGENCY MEASURES, INCLUDING OXYGEN, IVAS CLINICALLY INDICATED.